Recent news (Blog)
Cerebral venous sinus thrombosis: Clinical and neuroradiological analysis of 10 consecutive cases at Hospital México between January and December 2013.
- Hits: 2778
Por: Rosales Bravo, Luis Guillermo 1 y Torrealba Acosta, Gabriel 2.
1 Department of Neurology. Hospital México. Caja Costarricense de Seguro Social. San José, Costa Rica.
2 Department of Neurology. Hospital del Trauma. Instituto Nacional de Seguros. San José, Costa Rica.
Abstract: Cerebral venous sinus thrombosis (CVST) is an uncommon and potentially life-threatening neurological emergency. The annual incidence is estimated at 2-7 cases/million population. The sudden occlusion with a clot into a venous sinus causes an acute increase of the intracranial pressure rising to intracranial hypertension. Due to the rupture of cortical veins both parenchymal brain hemorrhage and subarachnoidal hemorrhage can be present in the initial unenhanced brain CT scan. Its diagnosis can be a challenge. High clinical suspicious is mandatory for an early diagnostic. Nowadays, with the use of CT and CTV its diagnosis is less difficult. Most of the patients recover without any neurological impairment. In CVST the rapid initiation of anticoagulant treatment is mandatory in order to reopen the occluded venous sinus. Neither the parenchymal brain hemorrhage nor the subarachnoidal hemorrhage contraindicated the anticoagulation. The main demographics, risk factors, clinical findings, neuroradiological characteristics, treatment and outcome of 10 consecutive patients with the diagnosis of CVST in a single public medical center in Costa Rica are described.
Key words: sinus thrombosis intracranial, cerebral hemorrhage, tomography X-Ray computed, anticoagulants. Source: Decs
Abbreviations
CVST: cerebral venous sinus thrombosis
MR: magnetic resonance
MRV: magnetic resonance venography
CT: computed tomography
CTV: computed tomography venography
VKA: vitamin K antagonist
mRS: modified Rankin scale
LMWH: low molecular weight heparin
INTRODUCTION
Cerebral venous sinus thrombosis is an uncommon and potentially life-threatening neurological emergency. CVST represents about 0.5-1% of all strokes [1]. The annual incidence is estimated at 2- 7 cases/million population. Young and middle aged women are more commonly affected, most of them having between 20 and 40 years of age [2].
The sudden occlusion by a clot in a venous sinus causes an acute increase of intracranial pressure and may lead to intracranial hypertension. Most frequently, the patient will have headache, but also there could be neurological deficits, seizures and depressed consciousness. Due to the rupture of cortical veins both brain parenchymal and subarachnoidal hemorrhage could be present on the initial unenhanced brain CT scan [3, 4]. However, in approximately 10% of the patients, the unenhanced brain CT scan could be normal [3].
In those cases, the diagnosis is certainly more challenging and requires of a greater clinical suspicious for an early diagnostic. Also, the implementation of neuroimaging techniques such as the CTV and MRV contribute to the diagnostic approach [5]. Finally, rapid implementation of anticoagulant treatment allows most of the patients to have a good prognosis and recovery without any neurological impairment.
SUBJECTS AND METHODS
Methods
Between January and December consecutives patients 2013 were analyzed with a diagnosis of CVST according to CTV reports. Information regarding patient’s gender, age, risk factors, clinical presentation, and findings on neurological examination, time elapsed between the initial manifestation and the definitive diagnosis, main findings on unenhanced brain CT scan and CTV, anticoagulation treatment, anticonvulsive therapy, in hospital complications, clinical outcome and in- hospital mortality were collected also.
Subjects
All patients with a diagnosis of CVST that were admitted to the departments of Neurology, Internal Medicine and the Intensive Care Unit at Hospital México in San José, Costa Rica were included. This study was approved by Institucional Scientific Ethics Committee of Costarricense de Seguro Social (CECI-CCSS)protocol number R017-SABI-00114).
CTV acquisition protocol
For the adquisition of the CTV images at Hospital México, the X-Ray Department uses a Philips Brillaince Diamond Select® (Koninklijke Philips N.V., Netherlands) CT-16 slice scanner with a high- performance system and a helicoidal technique. Each slice had a thichness of 0,625 mm to obtain both 2D and 3D reconstruction. The contrast administration was done with a bolus of 60 ml with 300 mg/ml and a flow velocity of 4 ml/s.
Data collection
For each patient data related to: gender, age and risk factors that included recent used of oral contraceptives (OC), pregnancy/puerperium, sinus infection, and patient ́s diagnosis of thrombophilia were gathered. Also information about clinical manifestations such as altered consciousness, headache, seizures, dysarthria, paresis, vertigo and visual deficits were collected. Findings on neurological examination among them depressed consciousness, papilledema; oftalmoparesis and limb paresis were documented.
Additionally, data concerning the time elapsed between the initial manifestation and the definitive diagnosis were obtained and also the main findings on unenhanced brain CT scan and CTV were registered, the anticoagulation treatment employed (enoxaparin vs. warfarin) as well as the anticonvulsive therapy used. Besides, the main in- hospital complications (pneumonia, urinary tract infection, convulsive status, need for decompressive surgery and lumbo-peritoneal shunt), the 6-moths clinical outcome according the mRS and in-hospital mortality were listed.
Laboratory investigations
White cell and platelet count, hemoglobin, baseline prothrombin time, activated partial thromboplastin time and International Normalized Ratio (INR) were analyzed for each patient. Serum chemistry including fasting sugar, blood urea nitrogen, serum creatinine, bilirubin, transaminase, protein, albumin, and serum electrolytes were also documented. Additionally test of thrombophilia with antinuclear antibodies (ANA), anti-dsDNA, antiphospholipid antibodies, lupus anticoagulant, Protein C, Protein S, factor V Leiden mutation, antithrombin III gene mutations and and MTHRF gene polymorphisms were screened.
Treatment
Treatment with low molecular weight heparin (LMWH, enoxaparin) was started immediately after establishing the diagnosis of CVST. The standard dose for full anticoagulation (1mg/kg every 12 hours subcutaneously) was used. Following the initial LMWH treatment, some patients were switched to warfarin for at least 6 months, aiming for an INR value between 2 and 3. Patients that manifested with seizures received antiepileptic treatment with phenytoin with an initial dose of 20mg/kg IV, followed by 300 mg/d po.
Outcome
The clinical outcome was measured 6 months after the patients’ discharge using the mRS: 0 - No symptoms.1 - No significant disability. Able to carry out all usual activities, despite some symptoms. 2 - Slight disability. Able to look after own affairs without assistance, but unable to carry out all previous activities. 3 - Moderate disability. Requires some help, but able to walk unassisted. 4 - Moderately severe disability. Unable to attend to own bodily needs without assistance, and unable to walk unassisted. 5 - Severe disability. Requires constant nursing care and attention, bedridden, incontinent and 6 - dead.
RESULT
10 patients were enrolled; all of them were females with an average age of 31 ± 6.7 years. Four of the patients were using OC, with an average time of use of 1-3 months before the CVST event. No cases were related to pregnancy/puerperium or sinus infection. Three cases had a gene mutation of the MTHRF (patients 1, 6 and 7). Patient 7 also had a factor V Leiden mutation. No other thrombophilia were found. Clinical manifestations included: headache (10 patients), seizures (4 patients), depressed consciousness (3 cases), visual deficits (3 patients) and vertigo (2 patients). Neurological examination documented: bilateral papiledema (3 patients), limb paresis (3 patients) and oftalmoparesis (1 patient). The median time elapsed between the initial clinical symptoms and the definitive diagnosis was of 9 days (IQR÷ 7-18 days). Table No. 1 shows the location of the parenchymal brain hemorrahage on the unenhanced brain CT scan and the occlude venous sinus on the CTV.
All patients had a thrombosis of the sagittal sinus (SSS), while the transverse sinus (TS) was the second most affected venous sinus. Half of the patients had thrombi on more than one venous sinus. All patients were treated with LMWH, four of them were switched to warfarin during their hospitalization and six continued treatment with enoxaparin. Anticoagulation was continued for at least 6 months. After that, the anticoagulation was stopped for all patients. However, patient 7 was kept on warfarin because of a double thrombophilia reported.
Table No. 1. Main findings in unenhanced brain CT scan and CTV

There were any hemorrhagic complications associated with anticoagulation therapy. One of the patient developed a bilateral papilledema that resulted in permanent blindness (Figure No. 1), after showing no improvement neither with the use of acetazolamide nor with a lumbar-peritoneal shunt that was placed to reduce the intracranial hypertension. Four patients had seizures as the initial clinical manifestation during the first two weeks. All of them were treated with phenytoin.
Table No. 2 shows the in-hospital complications and the clinical outcome measured with the mRS. Most of the patients had a good clinical outcome and there were no deaths registered.

Figure No. 1. Patient 7. Left eye fundoscopy that shows papilledema grade IV characterized by loss of major vessels around the optic disc. This patient developed a refractory ICH with bilateral blindness. A lumbo-peritoneal shunt was placed without obtaining a visual improvement.
Table No. 2. In-hospital complications and 6- month clinical outcome.

LPS indicates lumbo-peritoneal shunt; ICH: intracranial hypertension;
AMV: Assisted mechanical ventilation; BN: bronchopneumonia.
DISCUSSION
In this analysis all of the affected patients were women. Recent use of OCs and thrombophilia (gene mutation of the MTHRF and factor V Leiden mutation) were the two main risk factors associated with CVST. It is known that the recent use of OCs increases the risk of developing a CVST [6]. OCs have being described to increase the activity of most of the haemostatic factors, leading to systemic thrombosis [7]. This risk is higher when the patient that uses an OC has also an inherent prothrombotic condition [8]. Three patients with a mutation on the MTHRF gene were found. One patient had a factor V Leiden gene mutation, which is a common inherited thrombophilic disorder. This disorder causes a resistance to activated Protein C, which increases the risk to CVST [9]. All of our patients complained from headache. Headache is a manifestation of an increase in intracranial pressure, due to the occluded venous sinus. About 90 % of CVTS patients will refer headache [10].
In CVST headache is usually described as generalized and often progresses gradually into severity, over days to weeks. Few patients may present thunderclap headache (suggesting subarachnoid hemorrhage) or migrainous type headache [11]. Seizures are a common clinical manifestation in CVST. About half of the patients with CVST will have early seizures [12]. The seizures may be focal or generalized in type. Four patients in this analysis had early seizures. Patients with a hemorrhagic supratentorial lesion that involves the frontoparietal region are more likely to have a seizure [12]. Probably this is related with an irritating effect caused by the blood on the cortical surface. In this analysis three patients had a parenchymal hemorrhage located on the parietal region. All of these patients were treated with phenytoin. If the patient was seizure- free two years after the episode of CVST, the phenytoin was stopped. Neurological worsening after the diagnosis of CVST may occur. Patients with depressed consciousness on admission are more likely to deteriorate [13].
This neurological worsening could be related with a new parenchymal hemorrhagic lesion when neuroimaging is repeated. The most common visual deficit is visual loss. Severe visual loss due to CVST rarely occurs [14]. Visual loss is more likely to occur in patients with papilledema and those who present with increased intracranial pressure, as happened patient 7 in our analysis (Figure No. 1). In this case the visual loss was refractory to both medical and surgical treatment. Papilledema lead to optic atrophy and blindness. Delay in the diagnosis of CVST is common. High clinical suspicious is mandatory for an early diagnostic. In this analysis the median time elapsed between the initial clinical symptoms and the definitive diagnosis was 9 days.
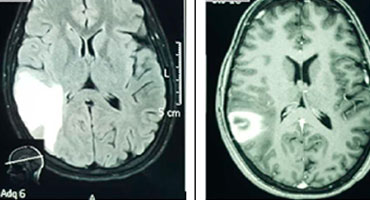
The use CTV is preferred to make the definitive diagnosis because it is quickly available and it has a high sensitivity and specificity to identify the affected venous sinus. CTV is similar to MRV in the diagnosis to CVST [15]. CT is commonly used as the initial neuroimaging test in patients with a clinical diagnosis of CVST. Unenhanced brain CT is often normal but may have findings that suggest CVST [16], for example the hyperattenuation of a dural sinus. Hyperattenuation in the dural sinuses on unenhanced brain CT scans have a high accuracy in the detection of acute cerebral sinus thrombosis. It reflect a newly formed thrombus [17]. Figure No. 2 shows some findings on unenhanced brain CT scan and CTV in our analysis. Figure No. 3 shows the relationship between an unenhanced CT and MRI in a case of a venous infarct.
All patients were treated with LMWH (enoxaparin) as soon as the diagnosis of CVST was established, using the standard doses for anticoagulation. According to Misra et al, LMWH resulted in significantly lower hospital mortality in CVST compared to unfractionated heparin [18]. Besides, LMWH has longer duration of action, has more stable therapeutic effect and does not require anticoagulation monitorization. Depending on the cause of the sinus thrombosis some patients were switched to warfarin, a vitamin K antagonist (VKA). The use of warfarin required the monitoring of INR often, besides this medicationhas pharmacological interaction with many other drugs or foods, leading to hemorrhagic complications.

Figure No. 2. Main findings on brain unenhanced CT scan and CTV. A (coronal) and B (sagittal) CTV shows an extensive filling defect on the SSS secondary to a clot (arrows, patient No. 6). C (axial, unenhanced) CT shows a left side parietal parenchymal hemorrhage (patient No. 5). This case had an occluded SSS and left TS. D (axial, unenhanced) linear hyperdensity on the left cortical parietal lobe secondary to a clot on a cortical vein (“cord sign”, arrow) (patient No. 8). This case had an occluded SSS.
Recently Geisbüsch et al reported that a novel factor Xa inhibitor (rivaroxaban) showed a similar clinical benefits as VKA in the treatment of CVST [19]. Rivaroxaban might be an alternative desirable treatment because of its known application, metabolism and its low rate of bleeding complications. Finally Table No. 2 shows in-hospital complications and clinical outcome of all patients.

Figure No. 3. Patient 4. A, B (axial) unenhanced brain CT scan shows a hypodense right parietal lesion with centered heterogeneous zone corresponding with a hemorrhage. C (axial, FLAIR) brain MRI shows hyperintense lesions compatible with edema secondary to a venous infarct. D (axial, T1WI post Gd) shows an enhanced zone on the same region. This patient had an occluded SSS (not shown).
Herniation attributable to unilateral mass effect is the major cause of death in CVST. Patient 1 developed a large left parieto-temporal parenchymal lesion caused herniation, that required of a decompresive hemicraniectomy surgery procedure was performed as lifesaving measure. A severe neurological condition, such as in these case, should not discourage surgery, because at least one-third of such patients can have a good recovery [20].
CONCLUSIONS
In this cases analysis all of the affected patients were women. Recent use of OCs and thrombophilia (gene mutation of the MTHRF and factor V Leiden mutation) were the two main risk factors associated with CVST. Most common occluded sinus was the SSS. The median time elapsed between the initial clinical symptoms and the definitive diagnosis was of 9 days. All the patients were treated with enoxaparin. Few of them were switched to warfarin. Finally most of the patients had a good clinical outcome and there were no deaths registered.
FUNDING SUPPORT: Caja Costarricense de Seguro Social (CCSS).
CONFLICT OF INTEREST STATEMENT
There is not conflict of interest to declare.
REFERENCES
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6:162–170.
2. Ferro JM, Canha ̃o P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664–670.
3. Wasay M, Bakshi R, Bobustuc G, Kojan S, Sheikh Z, Dai A, Cheema Z. Cerebral venous thrombosis: analysis of a multicenter cohort from the United States. J Stroke Cerebrovasc Dis. 2008;17:49 –54.
4. Verma R, Sahu R, Lalla R. S. Subarachnoid haemorrhage as the initial manifestation of cortical venous thrombosis. BMJ. Case Reports 2012:bcr2012006498. Doi:10.1136/bcr-2012- 006498.
5. Bonneville F. Imaging of cerebral venous thrombosis. Diagnostic and Interventional Imagin. 2014; 95:1145-1150.
6. Martinelli I, Sacchi E, Landi G, Taioli E, Duca F, Mannucci PM. High risk of cerebral-vein thrombosis in carriers of a prothrombin-gene mutation and in users of oral contraceptives. N Engl J Med. 1998;338: 1793–1797.
7. Vandenbroucke JP,Rosing J,Bloemenkamp KW,et al. Medical progress: oral contraceptives and the risk of venous thrombosis. N Engl J Med. 344 (2001), pp. 1527-1535.
8. De Freitas GR, Bogousslavsky J. Risk factors of cerebral vein and sinus thrombosis. Front Neurol Neurosci. 2008;23:23–54.
9. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood. 2006;107:2766 –2773.
10. Khealani BA, Wasay M, Saadah M, Sultana E, Mustafa S, Khan FS, Kamal AK. Cerebral venous thrombosis: a descriptive multicenter study of patients in Pakistan and Middle East. Stroke. 2008;39:2707–2711.
11. Cumurciuc R, Crassard I, Sarov M, Valade D, Bousser MG. Headache as the only neurological sign of cerebral venous thrombosis: a series of 17 cases. J Neurol Neurosurg Psychiatry. 2005;76:1084 –1087.
12. Kalita J, Chandra S, Kant Misra U . Significance of seizure in cerebral venous sinus thrombosis. Seizure. 2012; 21: 639-642.
13. Crassard I, Canha ̃o P, Ferro JM, Bousser MG, Barinagarrementeria F, Stam J. Neurological worsening in the acute phase of cerebral venous thrombosis in ISCVT (International Study on Cerebral Venous Thrombosis). Cerebrovasc Dis. 2003;16(suppl 4):60. Abstract.
14. Baumgartner RW, Studer A, Arnold M, Georgiadis D. Recanalisation of cerebral venous thrombosis. J Neurol Neurosurg Psychiatry. 2003;74: 459–461.
15. Ozsvath RR, Casey SO, Lustrin ES, Alberico RA, Hassankhani A, Patel M. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol. 1997;169:1699 –1707.
16. Ford K, Sarwar M. Computed tomography of dural sinus thrombosis. AJNR Am J Neuroradiol. 1981;2:539 –543.
17. Buyck PJ, De Keyzer F, Vanneste D, Wilms G, Thijs V, Demaerel P. CT Density Measurement and H:H Ratio Are Useful in Diagnosing Acute Cerebral Venous Sinus Thrombosis. AJNR Am J Neuroradiol. 2013 Aug; 34:1568-72.
18. Misra UK, Kalita J, Chandra S, Kumar B, Bansal V. Low molecular weigth heparin versus unfractionated heparin in cerebral venous sinus thrombosis: a randomized controlled trial. European Journal of Neurology. 2012,19: 1030-1036.
19. Geisbüsch M, Richter D, Herweh C, Ringleb P, Nagel S. Novel Factor Xa Inhibitor for the Treatment of Cerebral Venous and Sinus Thrombosis First Experience in 7 Patients. Stroke. 2014;45:2469- 2471.
20. Ferro JM, Crassard I, Coutinho JM, Canhao Patrícia, Barinagagarrementeria F, Cucchiara Brett, et al. Decompressive Surgery in Cerebrovenous Thrombosis: A Multicenter Registry and a Systematic Review of Individual Patient Data. Stroke. 2011;42:2825-283.

